Airway Repair and Regeneration
Our airway repair and regeneration program develops regenerative medicine approaches for life-threatening lung disease such as pulmonary fibrosis, cystic fibrosis and end-stage lung diseases of all types. These approaches include cell-based therapies as well as whole organ regeneration tissue engineering.
Project 1: Cell-Based Therapeutic Approaches for End-Stage Lung Disease
To apply regenerative medicine approaches to lung disease we need large number of cells of appropriate lineages. And for the whole organ approach, scaffolds are also needed for the cells to engraft and repopulate. As such, central to our research is the use of appropriate cell sources for use in our proposed strategies.
Induced progenitor-like cells - We have been working on the characterization and application of a novel cell type, which have termed induced progenitor-like (iPL) cells. These cells are an intermediate product of reprogramming on the path to pluripotency, have proliferative potential and retain epigenetic memory of cell of origin; thus allowing for lineage restricted differentiation in expanded numbers (Guo et al., 2017 and Guo et al., 2018). We have generated and characterized lung iPL cells from Club cells of the proximal airways and Alveolar type II epithelial cells of the distal lung and have demonstrated their utility in mouse models of cystic fibrosis and idiopathic pulmonary fibrosis (IPF). Our recent studies have shown shown that the iPL process can reverse cellular hallmarks of aging and restore the diminished clonogenic ability of aged lung epithelial cells derived from old mice. This finding is critical for older individuals affected by lung disease as the efficacy of donor cells decreases with a patient’s age. We are continuing this research by investigating the use of rejuvenated iPL cells derived from aged mice to treat IPF and have begun to evaluate this effect in human lung epithelial cells.
Molecularly engineered pluripotent-derived airway cells - One of the major challenges of using a patient’s own cells for treatment is the time, effort and cost required to obtain, reprogram and expand the cells. A universal therapeutic cell source that could be transplanted into any patient without triggering the immune system would be highly advantageous. Pluripotent stem cells hold enormous promise in this area, but there remain significant hurdles in their safety and acceptance by the recipient immune system. In collaboration with Dr. Andras Nagy, we are investigating the use of “designer” pluripotent stem cells which have an inducible trigger that allows elimination of proliferative cells cells to prevent uncontrolled cellular replication. Additional genetic modification to express immunomodulatory genes, prevents immune recognition in the recipient thus creating a universal donor cell that could serve all patients. We are currently exploring the utility of these “designer” pluripotent stem cells in the lung specifically evaluating whether their growth and replication can be controlled in a mouse model of IPF. Ultimately, our objective is to assess the feasibility of using these “designer” pluripotent stem cells to treat IPF.
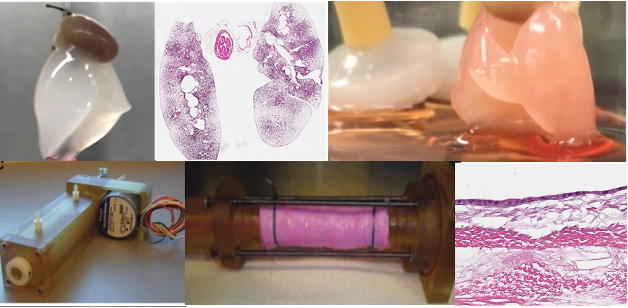
A continuous research theme in our lab is the use of tissue engineering approaches in combination with stem cells for generation of functional tissue such as the trachea and lung. In these studies, cells are seeded on biological scaffolds, which we obtain via detergent-based decellularization to remove resident cellular material, and the new recellularized scaffold is evaluated for cellular coverage and function with the ultimate goal for replacement of damaged tissue.
Project 2: Tissue Engineering Approaches for Airway Regeneration
Bioreactor-based recellularization of mouse lung scaffolds - In the lung, we are currently working on enhancing recellularization approaches in small animal and pre-clinical models. This work involves evaluating bioreactor parameters such as negative pressure and ventilation to optimize recellularization conditions. As with our cell-based therapy studies, we use pluripotent cell sources for tissue engineering applications. Currently, the team is evaluating the feasibility of iPL cells and the “designer” pluripotent stem cells described previously improves recellularization. This work will be continued to be done in collaboration with Drs. Amon and Bazylak at the University of Toronto and will include optimization of bioreactor technology and use of in silico modelling approaches to address physiologically relevant parameters such as ventilation and perfusion flow.
Generation of tissue-engineered airway grafts and tracheal constructs - Traumatic injury, stenosis, and malignancy involving large segments of the airway are very difficult to reconstruct. Tissue engineering provides a potential avenue for airway regeneration via creating functional constructs with the potential to repair and regenerate damaged airway tissue. Over the past several years, we have been working on development of functional airway epithelial grafts focusing on generation of small airway patches using human induced pluripotent-derived airway progenitor cells cultured on collagen-vitrigel membrane coated silk-fibroin hydrogels. Silk-fibroin based grafts are flexible with structural strength and support epithelial cell growth and differentiation with mucociliary function. In vivo studies in pigs showed that grafts are viable with cells present at 3 days after airway patch transplantation. Our current work focuses on evaluating stem-cell derived airway patches transplanted in vivo for a longer period of time. We are also working on tissue engineering approaches to enhance the functionality of grafts via induction of ciliary alignment. In parallel studies, we have begun to create large segment chimeric grafts that have been denuded of donor epithelium and re-epithelialized with induced pluripotent stem cell-derived airway progenitors. Repopulation is carried out in a biomimetic bioreactor system which allows for simultaneous exposure to air and liquid. Re-epithelialized grafts will ultimately be transplanted and evaluated for function.